Biogeochemical Platinum Cycling
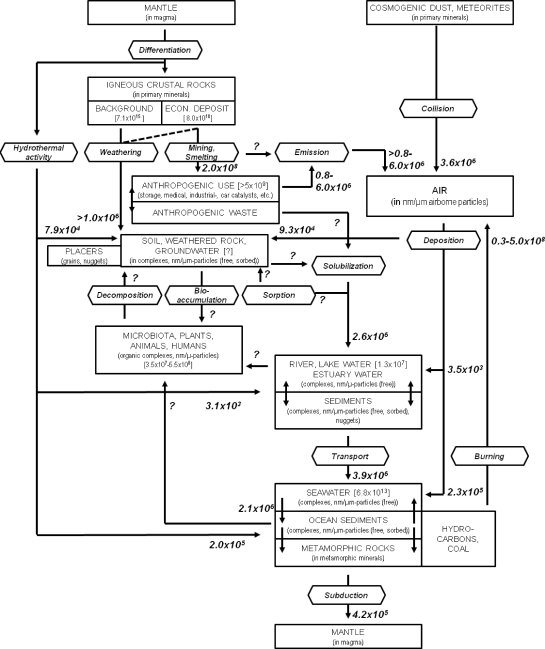
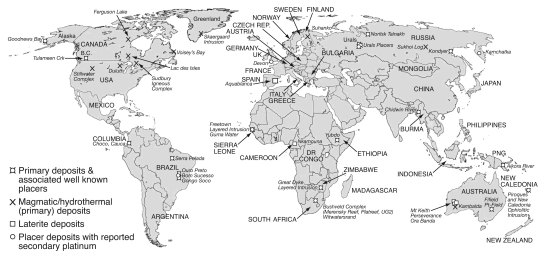
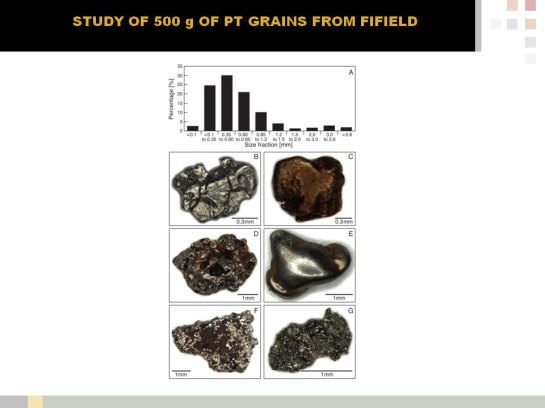
Platinum (Pt) is a very rare precious metal, which is quickly becoming a strategic commodity for industries in many countries. The demand for the Pt is ever increasing, due to its important role in the catalytic conversion of CO, HC and NOx in modern automobiles, its uses as catalyst in the petrochemical industry, as investment commodity and in medical applications, such as prosthetics and anti-cancer drugs. The increasing use of Pt, especially in vehicle catalysts, has led to the environmental dispersion of Pt nano- and micro-particles. Hence, soils and sediments in industrialized and urban areas and along main roads are commonly enriched in Pt, which can lead to bioaccumulation and toxic effects of Pt in animals and humans. Platinum supplies, however, are largely controlled by only two countries, South Africa and Russia, which currently produce more than 90 % of Pt and host more than 95.9 % of known reserves, as well as much of the expertise required for successful exploration, processing and metallurgy of platinum group elements (PGEs). To successfully explore for new Pt deposits, process ores and deal with possible ecotoxicological effects of Pt mining and usage, the fundamental processes and pathways of Pt dispersion and re-concentration in surface environments need to be understood. The aim of this research is to develop a process model for Pt cycling in the supergene environment. In order to achieve this, we integrate knowledge from geological/(biogeo)chemical research, with its focus on naturally occurring Pt and Pt mobility in and around Pt deposits, with the environmental/ecotoxicological research, which focuses mostly on anthropogenic Pt dispersion. In primary and secondary Pt deposits, Pt occurs as sulfide-, telluride- and arsenide minerals, native metal and alloyed to other PGEs and iron (Fe). In surface environments these minerals as well as anthropogenic Pt particles are transformed by physical and (bio)geochemical processes, leading to the mobilisation of Pt and other PGEs. Iron-, sulfur (S)-oxidising- and actinobacteria are known to produce thiosulfate, which form thermodynamically stable complexes with Pt. Ammonium, cyanide, low molecular weight organic acids (LMWOAs) and siderophores released by bacteria, fungi and plants as well as fulvic and humic acids and chloride also form stable complexes with Pt, increasing Pt mobility. Iron-oxides, clays and soil organic matter (SOM) is known to sequester Pt-complexes and –particles, in addition are microbes and plants capable of bioaccumulating and reductively precipitating mobile Pt compounds. Platinum nuggets are transformed under surface conditions, so are nuggets refined via the solubilisation of associated PGEs and Fe. In addition, oxygen-bearing Pt-rich phases and secondary Pt may form. For instance has (bio)mineralization in organic matter-rich sediments at Brazilian placer sites led to the formation of secondary Pt grains. Ultimately, surface cycling of Pt concludes in deep oceanic sediments, where Pt is re-concentrated in manganese (Mn) oxides formed as a result of microbial biomineralisation. When Pt-rich sediments are subducted, Pt re-enters the magmatic cycle. In conclusion, our research shows that geological, geochemical as well as biological and most recently anthropological processes are strongly interlinked in driving the biogeochemical cycling of Pt in Earth’ surface environments.
Reference: Reith F, Campbell SG, Ball AS, Pring A, Southam G (2014) Platinum in Earth Surface Environments. Earth-Science Rev. 131, 1-21.